PRODUCT OVERVIEW



Directions for use
Take an appropriate amount of the product in the palm of your hand and apply it gently, massaging the face until absorbed.
Precautions
– Please keep out of reach of infants to avoid accidental ingestion.
– Before use, conduct a local test to detect any discomfort. Please stop using it. immediately if any issue occurs.
Storage
Avoid direct sunlight and store at room temperature.
Ingredients
Water, Butanediol, Betaine, 1,2-Hexanediol Alcohol, p-Hydroxyacetophenone, Xanthan Gum, Glycerol, Glyceryl glucoside, Dipotassium Glycyrrhizinate, Hydroxypropyl Tetrahydropyran Triol, Dehydrated Xylitol, Tremella Fuciformis (TREMELLA FUCIFORMIS) Extract, Tetrahydromethylpyrimidine Carboxylic Acid.
Centella Asiatica Extract, Piper Methysticum Root Extract, Sodium Hyaluronate, Mung Bean (PHASEOLUS RADIATUS) Seed Extract, Xylitol, Wheat Keratin, B-Nicotinamide Mononucleotide, Dipeptide-1, Chloride Sodium, Dipeptidyl Diaminobutyroy|benzylamide Diacetate, EthyleneXyl Hexapeptide-8,
Tetrapeptide, Downhill Hydrogen Phosphate, Gentiana (GENTIANA SCABRA)
Extract, Decapeptide-4, Glycoprotein.
FAQ
NATIONAL PHARMACEUTUCAL REGULATORY AGENCY CERTIFICATE

IMU TEST REPORT
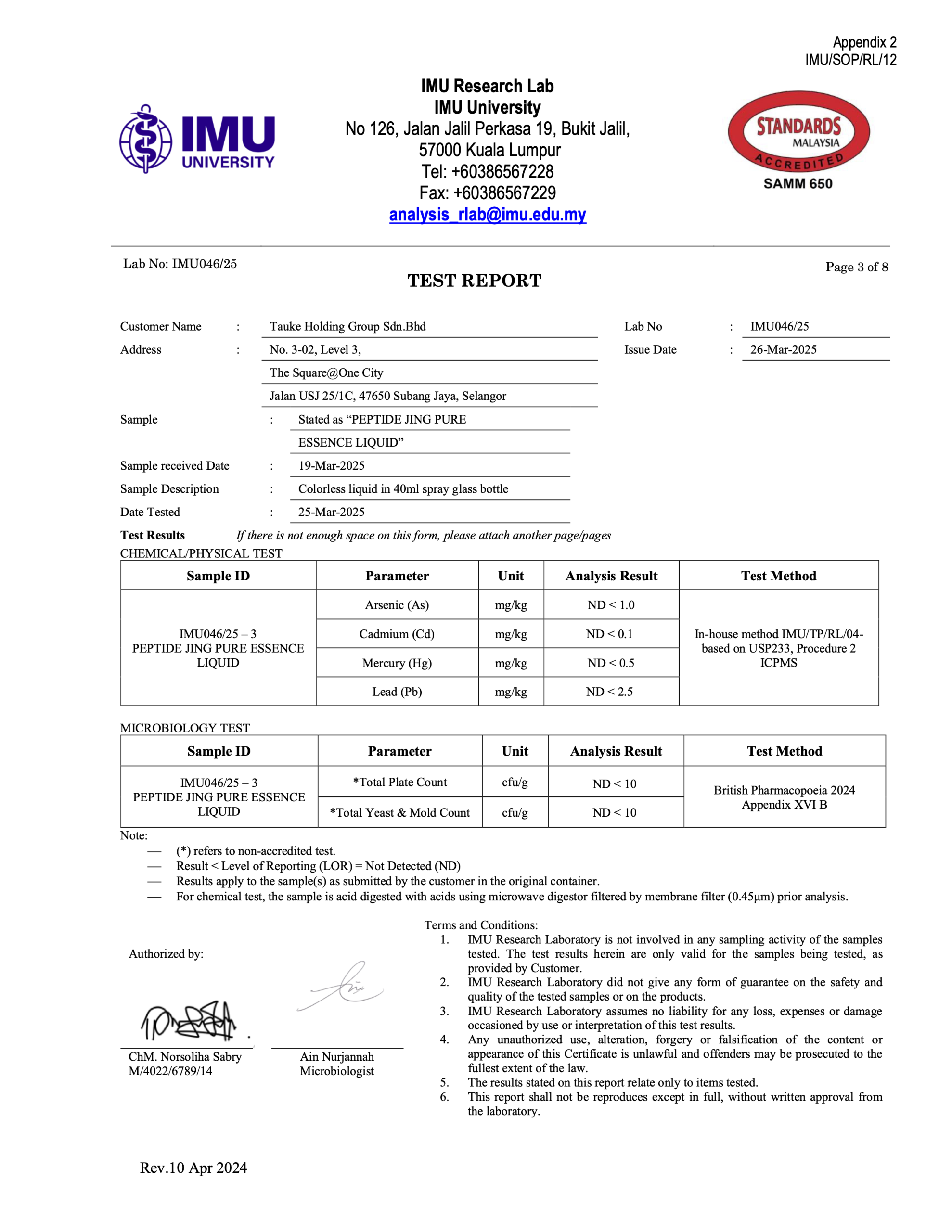
IMU Research Lab
IMU University
No 126, Jalan Jalil Perkasa 19, Bukit Jalil, 57000 Kuala Lumpur
Tel: +60386567228
Fax: +60386567229
Email: [email protected]
TEST REPORT
Customer Name: Tauke Holding Group Sdn. Bhd
Address: No. 3-02, Level 3, The Square@One City, Jalan USJ 25/1C, 47650 Subang Jaya, Selangor
Sample: Stated as “PEPTIDE JING PURE ESSENCE LIQUID”
Sample Received Date: 19-Mar-2025
Sample Description: Colorless liquid in 40ml spray glass bottle
Test Date: 25-Mar-2025
Issue Date: 26-Mar-2025
Test Results
(If there is not enough space on this form, please attach another page/pages)
CHEMICAL/PHYSICAL TEST
| Sample ID | Parameter | Unit | Analysis Result | Test Method |
|---|---|---|---|---|
| IMU046/25 – 3 PEPTIDE JING PURE ESSENCE LIQUID | Arsenic (As) | mg/kg | ND < 1.0 | In-house method IMU/TP/RL/04, based on USP233, Procedure 2, ICPMS |
| Cadmium (Cd) | mg/kg | ND < 0.1 | Same as above | |
| Mercury (Hg) | mg/kg | ND < 0.5 | Same as above | |
| Lead (Pb) | mg/kg | ND < 2.5 | Same as above |
MICROBIOLOGY TEST
| Sample ID | Parameter | Unit | Analysis Result | Test Method |
|---|---|---|---|---|
| IMU046/25 – 3 PEPTIDE JING PURE ESSENCE LIQUID | Total Plate Count | cfu/g | ND < 10 | British Pharmacopoeia 2024, Appendix XVI B |
| Total Yeast & Mold Count | cfu/g | ND < 10 | Same as above |
Note:
— (*) refers to non-accredited test.
— Result < Limit of Reporting (LOR) = Not Detected (ND)
— Results apply to the sample(s) as submitted by the customer in the original container.
— For chemical testing, the sample is acid digested with acids using a 0.45µm membrane filter prior to analysis.
Terms and Conditions:
IMU Research Laboratory is not involved in any sampling activity of the samples tested. The test results herein are only valid for the samples being tested, as provided by the Customer.
IMU Research Laboratory did not give any form of guarantee on the safety and quality of the tested samples or on the products.
IMU Research Laboratory assumes no liability for any loss, expenses or damage occasioned by use or interpretation of this test results.
Any unauthorized use, alteration, forgery or falsification of the content or appearance of this Certificate is unlawful and offenders may be prosecuted to the fullest extent of the law.
The results stated on this report relate only to items tested.
This report shall not be reproduced except in full, without written approval from the laboratory.
Overall Conclusion
- No heavy metals or microbial contaminants were detected in this sample, and all tested items met the required standards.
- The test results indicate that the product is safe, clean, and compliant with standards in all tested aspects.


